PharmaC’s OnSite IV took home bronze at a New York City contest.
By Jennifer Dorsey | Jackson Hole News & Guide

In the not-too-distant future people receiving drugs intravenously might use a product invented by a Jackson company.
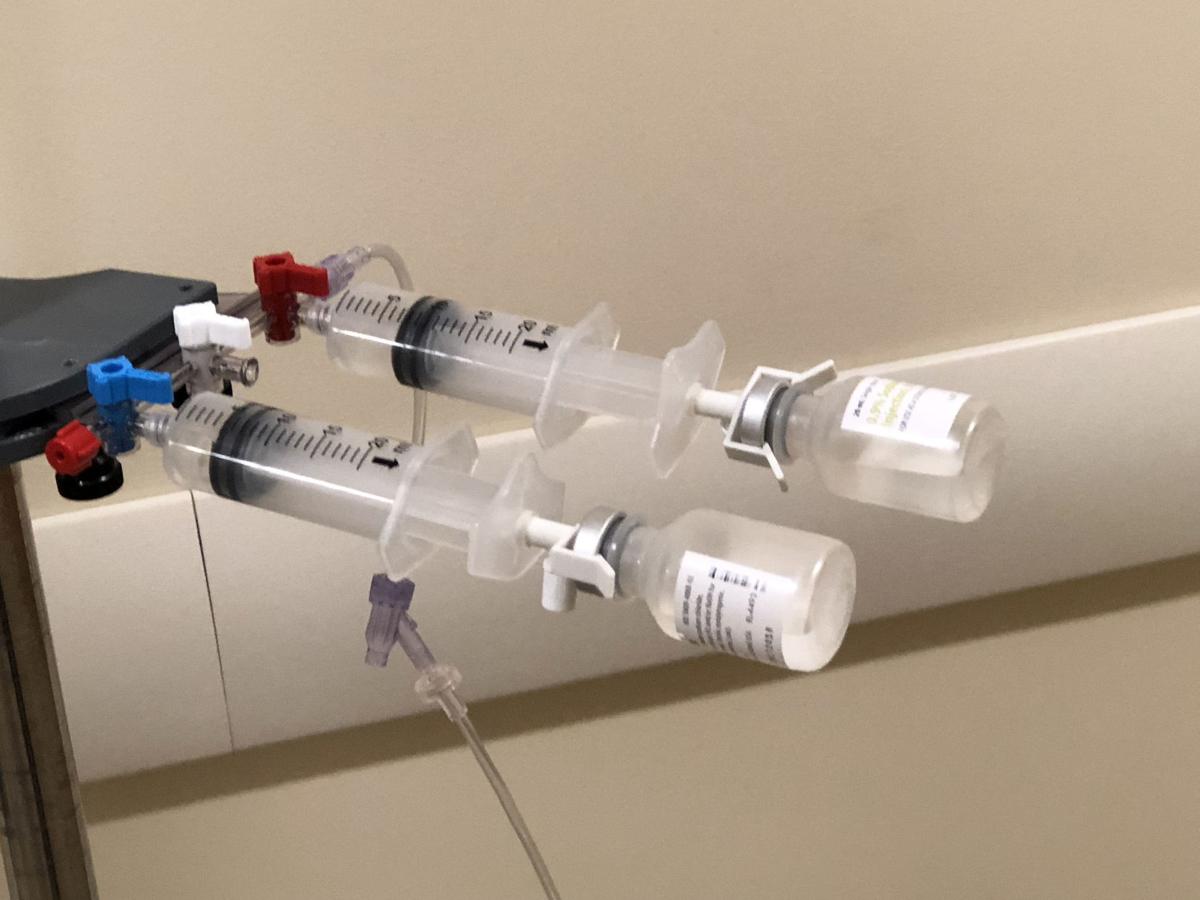
PharmaC LLC’s OnSite IV makes it quick and easy to mix or reconstitute a drug and deliver it into an IV bag, an elastomeric pump or a port on a patient’s body — all without using needles.
Last month the device won bronze in the Drug Delivery and Combination Products category of the Medical Design Excellence Awards in New York. Just being named a finalist was a coup, as the competition draws big pharmaceutical names like Johnson & Johnson and Eli Lilly and Company.
As a result, PharmaC has been receiving inquiries from around the word, CEO Chris Tice said.
“We anticipate the product to be on the market in six to 12 months,” Tice said. “There are companies that have expressed interest in manufacturing and distributing the device.”
The idea for the Onsite IV sprang from a business called Pharmacy Solutions that Tice ran with pharmacist Dave Pestotnik. It shipped intravenous medications to patients at home in a multistate area. Because some medications have a shelf life of only 24 or 48 hours, they saw a niche for a product that enabled on-site preparation.
“If a patient needs a week’s worth of medications you could only ship them a day or two days’ worth of medications, which is not feasible in rural locations,” Tice said.
Tice and Pestotnik sold Pharmacy Solutions to CVS a few years ago and are now partners in PharmaC and co-inventors of the Onsite IV.
They created rudimentary versions of the device out of cut-up syringes, pieces of wood and epoxy, ultimately developing a prototype. They put together a team that included a scientist, former Abbott engineers and people with health care and business experience.
A computer design led to a model created on a 3-D printer and then to molds for manufacturing the device so the U.S. Food and Drug Administration could take a look at it. They also showed it to doctors in Jackson Hole, who have given it positive reviews, Tice said.
PharmaC has three patents for the device. Tice and Pestotnik are on two. The third also names engineer Tim Reynolds. The FDA cleared the device in December 2016, Tice said.
A Jackson resident since he was 4, he is married to Carmel Tice, a pharmacist at St. John’s Medical Center. Their daughter, Amy Ciampi, is a pharmacist and district leader for Rite Aid in Bend, Oregon.
In addition to sitting down for an interview Chris Tice provided written responses to questions about the Onsite-IV:
Q: The Onsite-IV is described as a “closed-system needleless drug administration device.” Can you explain what that means in terms the average person outside the medical field could understand?
A: The closed-system allows the admixture of medications in a self-contained syringe. The syringe has a valve that permits the flow of a diluent, such as saline, to be transferred from the syringe body to the medication vial and then back to the syringe for administration.
Since the transfer and admixture is done within the syringe the risks for outside contamination are greatly reduced. There are stringent pharmacy and other regulatory requirements to ensure the admixture and dispensing of medications.
Q: What problem does the Onsite-IV solve?
A: There are numerous benefits and uses for the On-Site IV device. The primary benefits include the closed system feature, the elimination of needles in mixing, dispensing and transferring medications, the avoidance of multiple aspirations, and the elimination of labeling on syringes as the vial remains attached.
The On-Site IV has numerous applications, including multiple settings within the hospital for emergency room, patient care floors and surgery. The other applications encompass home care, skilled nursing facilities, physician offices and the military.
Q: How is the Onsite-IV different or better than comparable devices on the market?
A: The OnSite-IV device is unique in a number of its features. First, other comparable devices require a combination of separate components and additional steps to achieve the function of the OnSite-IV device. The use of the device in the surgical setting is unique, and there are no known comparable devices.
Q: Where did you get the idea?
A: During the operation of our home intravenous pharmacies, we had to develop a solution for extending the expiration of medications. There is a limited shelf life of prepared intravenous medications. We typically had to ship the intravenous medications throughout a multistate area. A process was needed whereby a clinician could add the diluent to the medication for patient administration.
Q: Is the Onsite-IV in production?
A: We have manufactured a limited quantity in order to obtain FDA clearance and for demonstration purposes.
Q: Where is it made?
A: The OnSite-IV device is manufactured in California.
Q: Who are you marketing it to first?
A: Our company, PharmaC LLC, is looking for a strategic partner to manufacture and distribute the product. We anticipate that there will be several partners, as the device covers distinct health care markets as mentioned above.
Q: What does the Bronze award mean for your business?
A: We are truly honored to have won a Bronze award at the 20th annual Medical Design and Excellence Awards in New York. Our company was in direct competition with some of the world’s largest pharmaceutical companies. We have garnered international interest in the distribution of our device.
Q: Do you have other medical devices on the drawing board?
A: We do have other future plans that include a prefilled syringe, the extension of different size devices and a chemo version.